RA Study
The efficacy of subcutaneous Remsima® in rheumatoid arthritis patients was assessed in a randomised, parallel-group pivotal Phase I/III study.1
Objective1,2
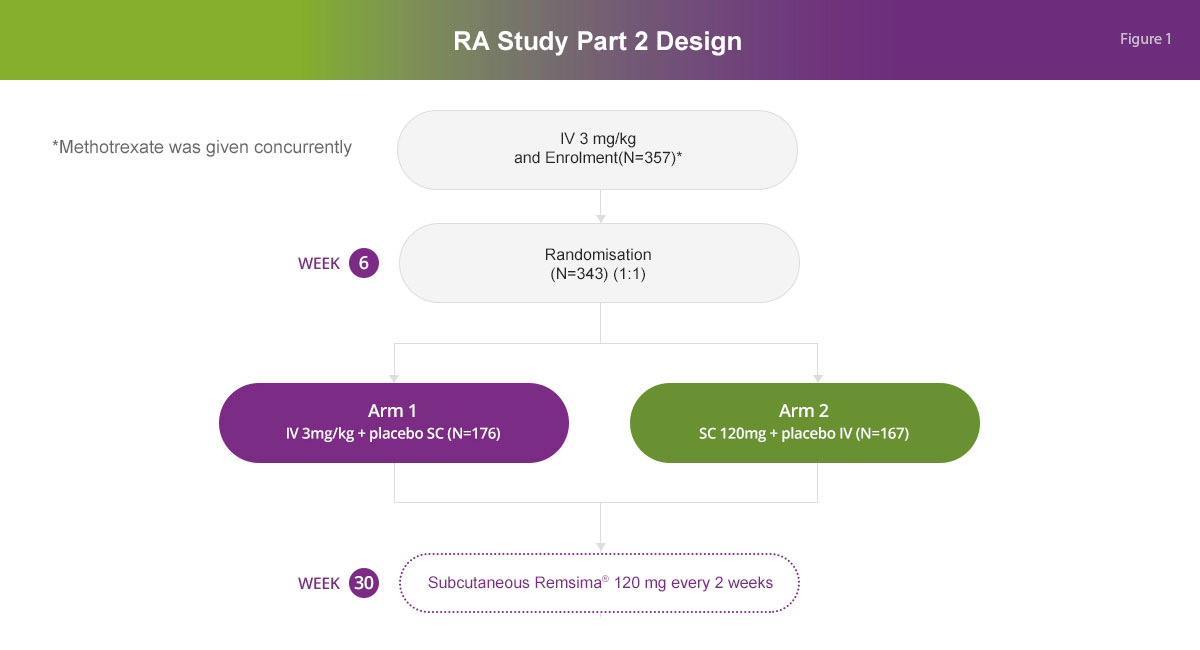
The study consisted of two parts: Part 1 to determine the optimal dose of subcutaneous infliximab and Part 2 to demonstrate noninferiority in terms of efficacy of subcutaneous infliximab compared to intravenous infliximab treatment, with the primary outcome being the mean change from baseline in DAS28-CRP at week 22.
Study design1,2
In Part 2 of this study, among 357 patients who were enrolled to receive 2 doses of Remsima® 3 mg/kg intravenously at Weeks 0 and 2, 167 patients were randomised to receive Remsima® 120 mg subcutaneously at Week 6 and every 2 weeks up to Week 54, while 176 patients were randomised to receive Remsima® 3 mg/kg intravenously at Weeks 6, 14 and 22 and then switched to Remsima® 120 mg subcutaneous at Week 30 once-every 2 weeks up to Week 54. Methotrexate was given concomitantly.
Results2
Efficacy
The primary endpoint of the study was the treatment difference of the change from baseline of DAS28 (CRP) at Week 22. The estimate of treatment difference was 0.27 with corresponding lower limit of the two-sided 95% confidence interval [CI] of 0.02 (95% CI: 0.02, 0.52), which was greater than the pre-specified non-inferiority margin of -0.6 indicating non-inferiority of Remsima® subcutaneous formulation to Remsima® intravenous formulation.1
The analysis of other efficacy endpoints showed that efficacy profile of Remsima® subcutaneous formulation compared to Remsima® intravenous formulation in RA patients was generally comparable in terms of disease activity measured by DAS28 (CRP and ESR) and ACR response up to Week 54. The mean scores for DAS28 (CRP) and DAS28 (ESR) gradually decreased from baseline at each time point until Week 54 in each treatment arm.1
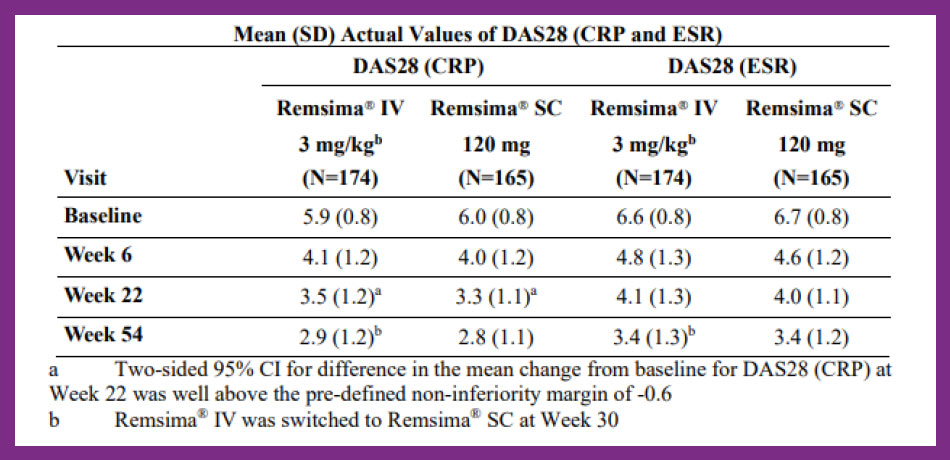
Table 13. Remsima® Australian Product Information.1
References
1. Remsima® Australian Product Information. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2020-PI-02540-1&d=202103141016933&d=20221121172310101. Last accessed: Nov 2022.
2. Westhovens R. et al., Efficacy, pharmacokinetics and safety of subcutaneous versus intravenous CT-P13 in rheumatoid arthritis: a randomized phase I/III trial. Rheumatology (Oxford). 2020 Nov 23:keaa580. doi: 10.1093/rheumatology/keaa580. Epub ahead of print.
Abbreviations
ADA, anti-drug antibody; DAS28-CRP, Disease Activity Score-28 with C-reactive protein; DAS28-ERP, Disease Activity Score-28 with erythrocyte sedimentation rate; IV, intravenous; RA, rheumatoid arthritis; SC, subcutaneous
Safety1,2
The incidence of systemic injection reactions (e.g. rash, pruritus, flushing and oedema) was 1.2 per 100 patient-years in the Remsima® subcutaneous group (from Week 6) and 2.1 per 100 patient-years in the Remsima® intravenous group who switched to Remsima® subcutaneous administration (from Week 30). All systemic injection reactions were mild to moderate.1,2
The incidence of localised injection site reactions (e.g. injection site erythema, pain, pruritus and swelling) was 17.6 per 100 patient-years in the Remsima® subcutaneous group (from Week 6) and 21.4 per 100 patient-years in those who switched to Remsima® subcutaneous administration (from Week 30). Most of these reactions were mild to moderate and resolved spontaneously without any treatment within a day.1,2
References
1. Remsima® Australian Product Information. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2020-PI-02540-1&d=202103141016933&d=20221121172310101. Last accessed: Nov 2022.
2. Westhovens R. et al. Efficacy, pharmacokinetics and safety of subcutaneous versus intravenous CT-P13 in rheumatoid arthritis: a randomized phase I/III trial. Rheumatology (Oxford). 2020 Nov 23:keaa580. doi: 10.1093/rheumatology/keaa580. Epub ahead of print.
Abbreviations
ADA, anti-drug antibody; DAS28-CRP, Disease Activity Score-28 with C-reactive protein; IV, intravenous; RA, rheumatoid arthritis; SC, subcutaneous
Immunogenicity
Immunogenicity was similar between the arms: 114 (67.9%; in the subcutaneous arm) and 129 (73.7%; intravenous arm) patients had at least one positive post-treatment ADA result. In both arms, the proportion of ADA-positive patients increased after the dose-loading phase.1
References
1. Remsima® Australian Product Information. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2020-PI-02540-1&d=202103141016933&d=20221121172310101. Last accessed: Nov 2022.
2. Westhovens R. et al., Efficacy, pharmacokinetics and safety of subcutaneous versus intravenous CT-P13 in rheumatoid arthritis: a randomized phase I/III trial. Rheumatology (Oxford). 2020 Nov 23:keaa580. doi: 10.1093/rheumatology/keaa580. Epub ahead of print.
Abbreviations
ADA, anti-drug antibody; DAS28-CRP, Disease Activity Score-28 with C-reactive protein; IV, intravenous; RA, rheumatoid arthritis; SC, subcutaneous


