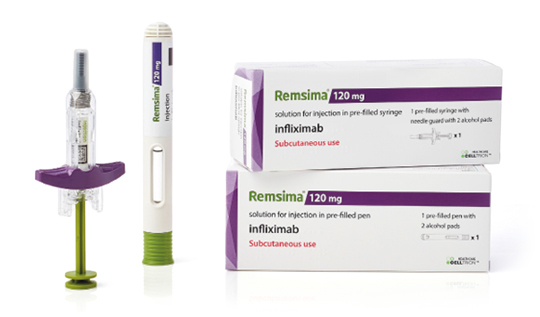
Remsima® provides a subcutaneous infliximab treatment option for patients with RA, CD, UC, AS, PsA and PsO based on comparable efficacy and safety to intravenous formulation.1,2,3*
*In patients with rheumatoid arthritis and inflammatory bowel disease1,2,3*
Authority required. Refer to PBS Schedule for full authority information.
Approved Indications
LEARN MORE
-

About Remsima®
LEARN MORE
-

Prescribing Remsima®
LEARN MORE
-

Clinical Data
LEARN MORE
-
Resources
LEARN MORE
Advise your patients to report any possible side effects, including effects not listed in the Consumer Medicine Information (CMI) or Product Information (PI).
You can report side effects directly to Celltrion Healthcare Australia (T: 1800 325 228)
and to the Therapeutic Goods Administration (TGA) in accordance with their requirements. (please visit https://aems.tga.gov.au/)
By reporting side effects, you can help provide more information on the safety of this medicine.
References
1. Remsima® Australian Product Information. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2020-PI-02540-1&d=202103141016933&d=20221121172310101. Last accessed: November 2023.
2. Schreiber S, Ben-Horin S, Leszczyszyn J, Dudkowiak R, Lahat A, Gawdis-Wojnarska B, Pukitis A, Horynski M, Farkas K, Kierkus J, Kowalski M, Lee SJ, Kim SH, Suh JH, Kim MR, Lee SG, Ye BD, Reinisch W. Randomized Controlled Trial: Subcutaneous vs Intravenous Infliximab CT-P13 Maintenance in Inflammatory Bowel Disease. Gastroenterology. 2021 Jun;160(7):2340-2353. doi: 10.1053/j.gastro.2021.02.068. Epub 2021 Mar 5. PMID: 33676969.
3. Westhovens R, et al. Rheumatology 2020: doi: 10.1093/rheumatology/keaa580
Abbreviations
AS, ankylosing spondylitis; CD, Crohn's disease; PsA, psoriatic arthritis; PsO, plaque psoriasis; RA, rheumatoid arthritis; UC, ulcerative colitis


 Rheumatoid Arthritis
Rheumatoid Arthritis  Moderate to severe and refractory fistulising Crohn’s Disease
Moderate to severe and refractory fistulising Crohn’s Disease Moderately to severely active Ulcerative Colitis
Moderately to severely active Ulcerative Colitis Active Ankylosing Spondylitis
Active Ankylosing Spondylitis Active and progressive Psoriatic Arthritis
Active and progressive Psoriatic Arthritis Moderate to severe Plaque Psoriasis
Moderate to severe Plaque Psoriasis