Administration
Guides for Storage1,2
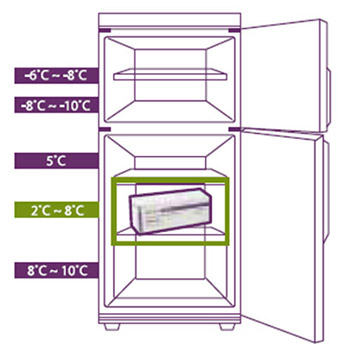
Keep Remsima® in the refrigerator (2o~8oC). Do not freeze.
Keep the product in its outer carton in order to protect from light.
Remsima® can be stored in its carton outside the refrigerator up to a maximum of 25℃ for a period of up to 28 days.
If it is stored outside the fridge, discard the medicine if not used within 28 days or expiry printed on the carton whichever is earlier.
Shelf life1,3,4
Shelf life: up to 36 months. Please check the expiration date on the packaging.
Instructions for Self-injection
You can refer to Instructions for Use in the consumer medicines information leaflet.
You can also refer to the Remsima® administration guide video or Information for use brochure for instructions.
Important Information
Use the syringe or pen ONLY if your healthcare provider has trained you on how to give an injection.
Ask your healthcare provider how often you will need to give an injection.
Rotate the injection site each time you give an injection. Each new injection site should be at least 3 cm away from the previous injection site.
Do not use the syringe if it has been dropped or is visibly damaged. A damaged syringe may not function properly.
Do not reuse the syringe or pen.
Do not shake the syringe or pen at any time.
-
 Instruction brochure for
Instruction brochure for
pre-filled pen
-
 Administration guide video
Administration guide video
for pre-filled pen
Instructions for pre-filled pen
-
 Instruction brochure for
Instruction brochure for
pre-filled syringe type
-
 Administration guide video
Administration guide video
for pre-filled syringe type
Instructions for pre-filled syringe types
Patient Support Program
For further support in administering Remsima®, you can contact our patient support program co-ordinator to receive further information and support, including Remsima® self-injection training. You can contact the program co-ordinator on 1800 782 288 on weekdays, excluding public holidays, between 9.00am - 5.00pm AEST.
Reference
1. Remsima® Australian Consumer Medicine Information (CMI) PF pen. Available at: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2021-CMI-01619-1 Accessed January 2023.
2. Remsima® Australian Consumer Medicine Information (CMI) PF syringe. Available at: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2021-CMI-01618-1 Accessed January 2023.
3. Therapeutic Goods Administration. Public Summary. REMSIMA infliximab 120 mg solution for injection prefilled syringe in auto-injector pen. Available at: https://www.ebs.tga.gov.au/servlet/xmlmillr6?dbid=ebs/PublicHTML/pdfStore.nsf&docid=326188&agid=%28PrintDetailsPublic%29&actionid=1. Accessed June 2024.
4. Therapeutic Goods Administration. Public Summary. REMSIMA infliximab 120 mg solution for injection prefilled syringe with safety guard. Available at: https://www.ebs.tga.gov.au/servlet/xmlmillr6?dbid=ebs/PublicHTML/pdfStore.nsf&docid=326187&agid=%28PrintDetailsPublic%29&actionid=1. Accessed June 2024.


