IBD Study
The efficacy of subcutaneous infliximab in active Crohn’s disease and active ulcerative colitis patients was assessed in an open-label, randomised, parallel-group, Phase I study.1,2
Objective1
Primary outcome measure: to demonstrate non-inferiority in terms of pharmacokinetics (PK) of subcutaneous Remsima® compared to intravenous Remsima® treatment, comparing observed pre-dose concentration (Ctrough) of Remsima® at week 22 in patients receiving the subcutaneous versus intravenous formulations. In addition, efficacy, pharmacodynamics, safety, and immunogenicity were evaluated up to week 54.
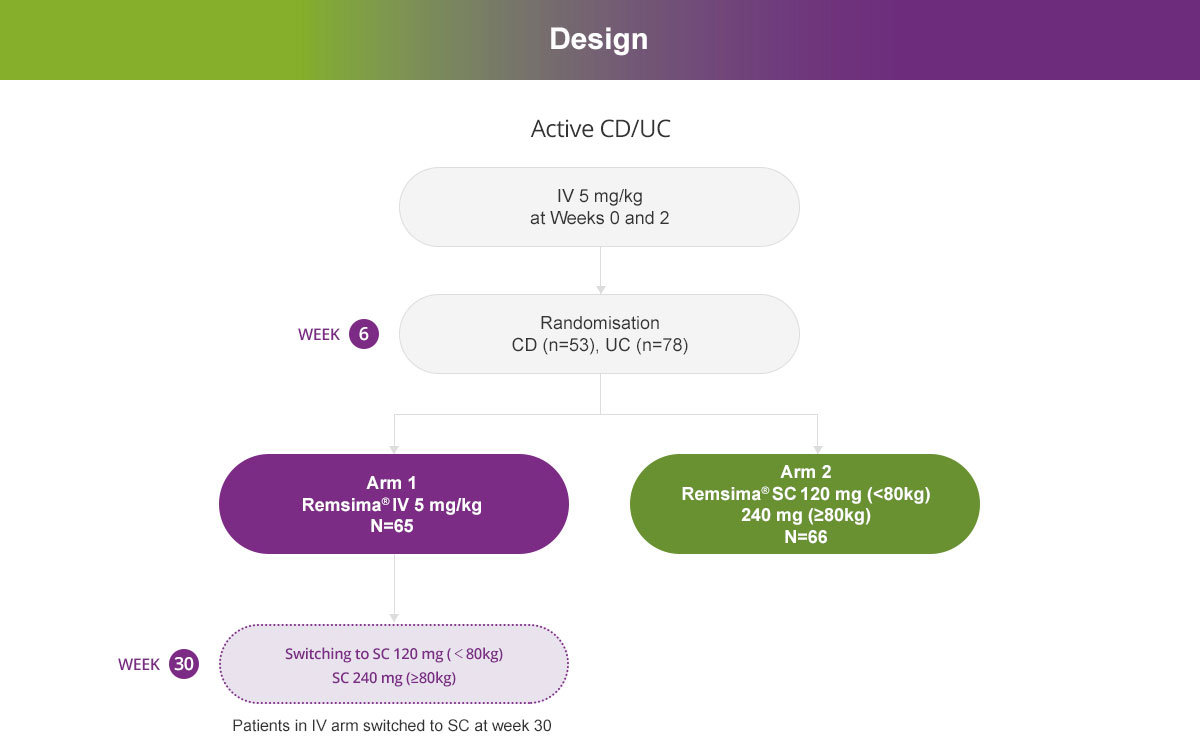
Study design1
- 66 patients (28 patients with active Crohn’s disease and 38 patients with active ulcerative colitis) were randomised to receive Remsima® 120 mg (or 240 mg if weight ≥80 kg) subcutaneously at Week 6 and every 2 weeks up to Week 54
- 65 patients (25 patients with active Crohn’s disease and 40 patients with active ulcerative colitis) were randomised to receive Remsima® 5 mg/kg intravenously at Week 6, 14 and 22 and then switched to Remsima® 120 mg (or 240 mg if weight ≥80 kg) subcutaneous formulation at Week 30 every 2 weeks up to Week 54.
A cohort of 136 patients (57 patients with active Crohn’s disease and 79 patients with active ulcerative colitis) received 2 doses of Remsima® 5 mg/kg intravenously at Weeks 0 and 2.
At week 6
Results1
Pharmacokinetics
The mean Ctrough (pre-dose drug concentration) was higher in the group receiving subcutaneous Remsima® than in those receiving intravenous Remsima® with a confidence level exceeding 80% meeting the primary outcome of non-inferiority of subcutaneous versus intravenous formulations.1
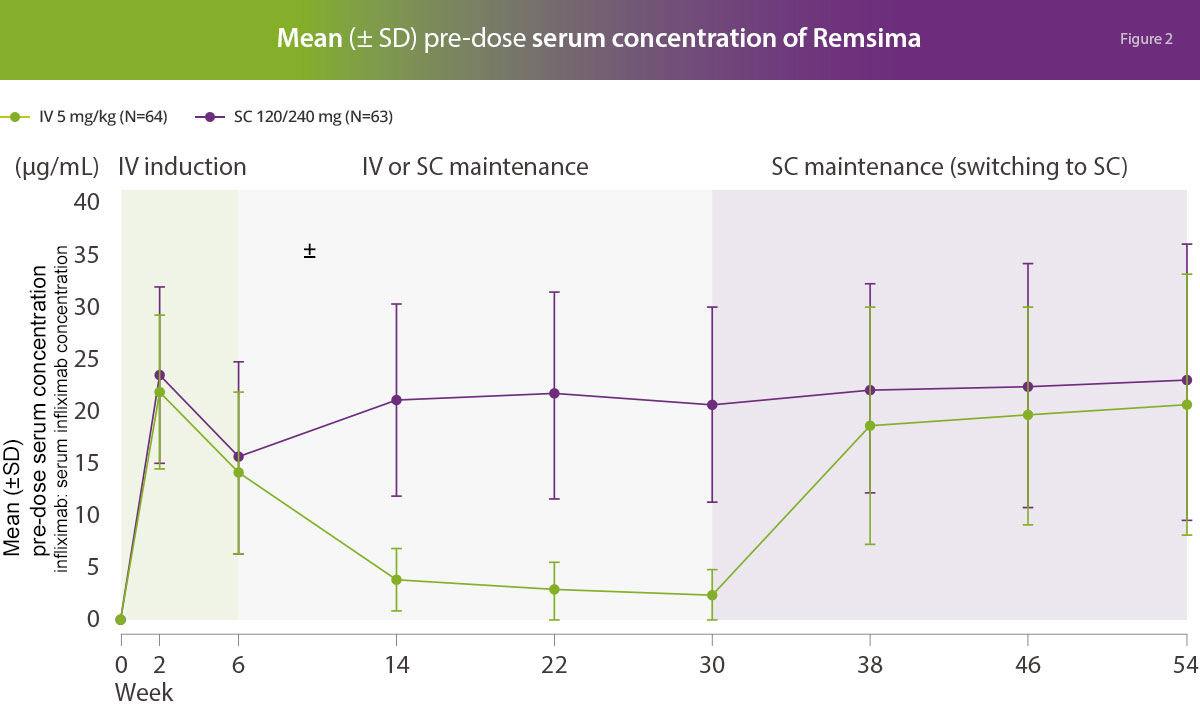
Six (9.1%) and 8 (12.3%) patients in the SC and IV arms, respectively, had at least one escalated SC dose from 120 to 240 mg on or after Week 30.
Adapted from Schrieber S, et al. Gastroenterology; accepted 2021.1
References
1. Schreiber S, Ben-Horin S, Leszczyszyn J, Dudkowiak R, Lahat A, Gawdis-Wojnarska B, Pukitis A, Horynski M, Farkas K, Kierkus J, Kowalski M, Lee SJ, Kim SH, Suh JH, Kim MR, Lee SG, Ye BD, Reinisch W. Randomized Controlled Trial: Subcutaneous vs Intravenous Infliximab CT-P13 Maintenance in Inflammatory Bowel Disease. Gastroenterology. 2021 Jun;160(7):2340-2353. doi: 10.1053/j.gastro.2021.02.068. Epub 2021 Mar 5. PMID: 33676969.
2. Remsima® Australian Product Information. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2020-PI-02540-1&d=202103141016933&d=20221121172310101. Last accessed: Nov 2022.
Abbreviations
CD, Crohn’s disease; CDAI, Crohn's Disease Activity Index; IBD, inflammatory bowel disease; IV, intravenous; PK, pharmacokinetics; SC, subcutaneous; SD, standard deviation; SES-CD, Simplified Endoscopic Activity Score for Crohn’s Disease; UC, ulcerative colitis
Clinical efficacy
In active Crohn’s disease patients, the efficacy results following Remsima® 120 mg subcutaneous formulation were generally comparable to Remsima® 5 mg/kg intravenous formulation in terms of clinical response (CDAI-70 response defined as a decrease in CDAI by ≥70 points and CDAI-100 response defined as ≥100 points from baseline), clinical remission (defined as an absolute CDAI score of <150 points) and endoscopy assessments (endoscopic response defined as a decrease in ≥50% of overall Simplified Endoscopic Activity Score for Crohn’s Disease (SES-CD) score from the baseline value and endoscopic remission defined as an absolute SES-CD score of ≤2 points).1
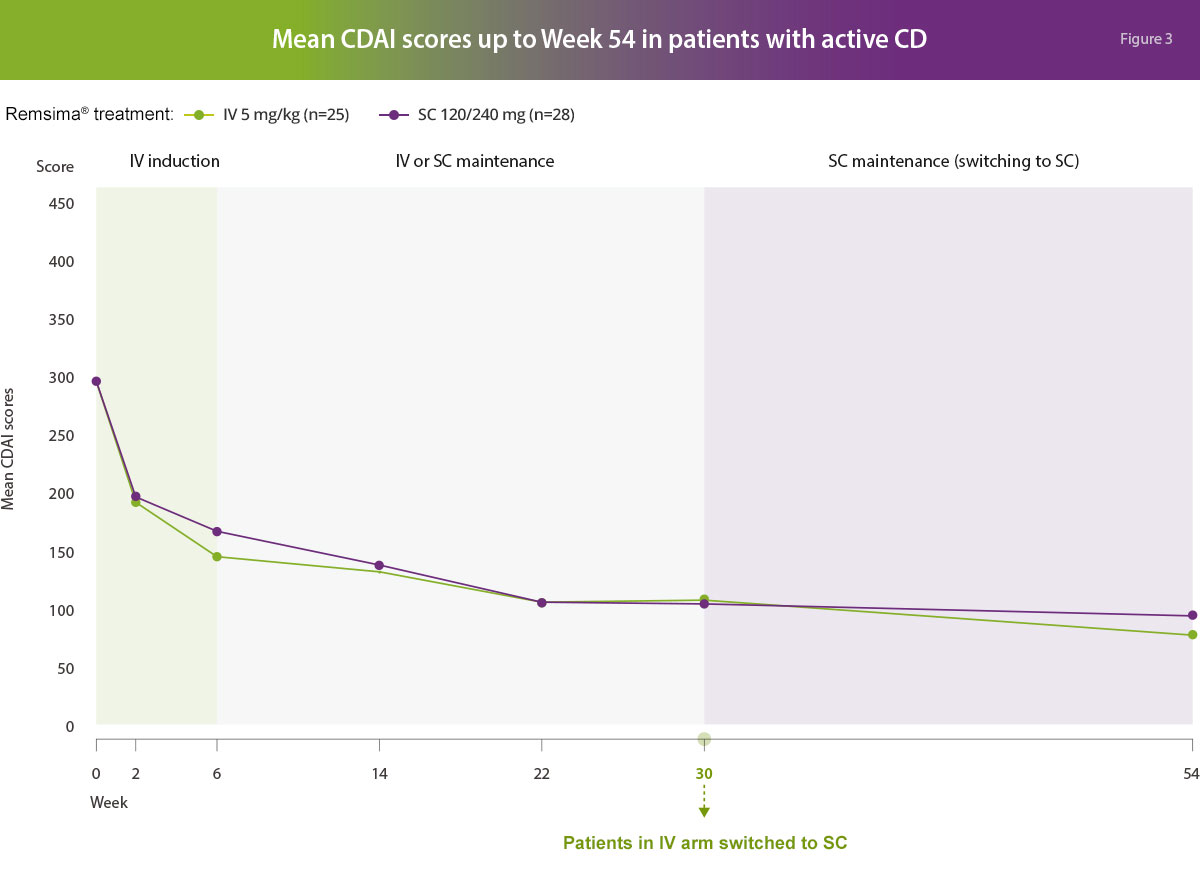
Adapted from Schrieber S, et al. Gastroenterology; accepted 2021.1
In active ulcerative colitis patients, the efficacy results following Remsima® 120 mg subcutaneous formulation were generally comparable to Remsima® 5 mg/kg intravenous formulation in terms of clinical response (defined as a decrease from baseline in total Mayo score of at least 3 points and at least 30% or a decrease from baseline in partial Mayo score at least 2 points, with an accompanying decrease from baseline in the subscore for rectal bleeding of at least 1 point, or an absolute subscore for rectal bleeding of 0 or 1), clinical remission (defined as a total Mayo score of ≤ 2 points with no individual subscore exceeding 1 point, or partial Mayo score of ≤1 point) and mucosal healing (defined as absolute endoscopic subscore of 0 or 1 from Mayo Scoring System).1
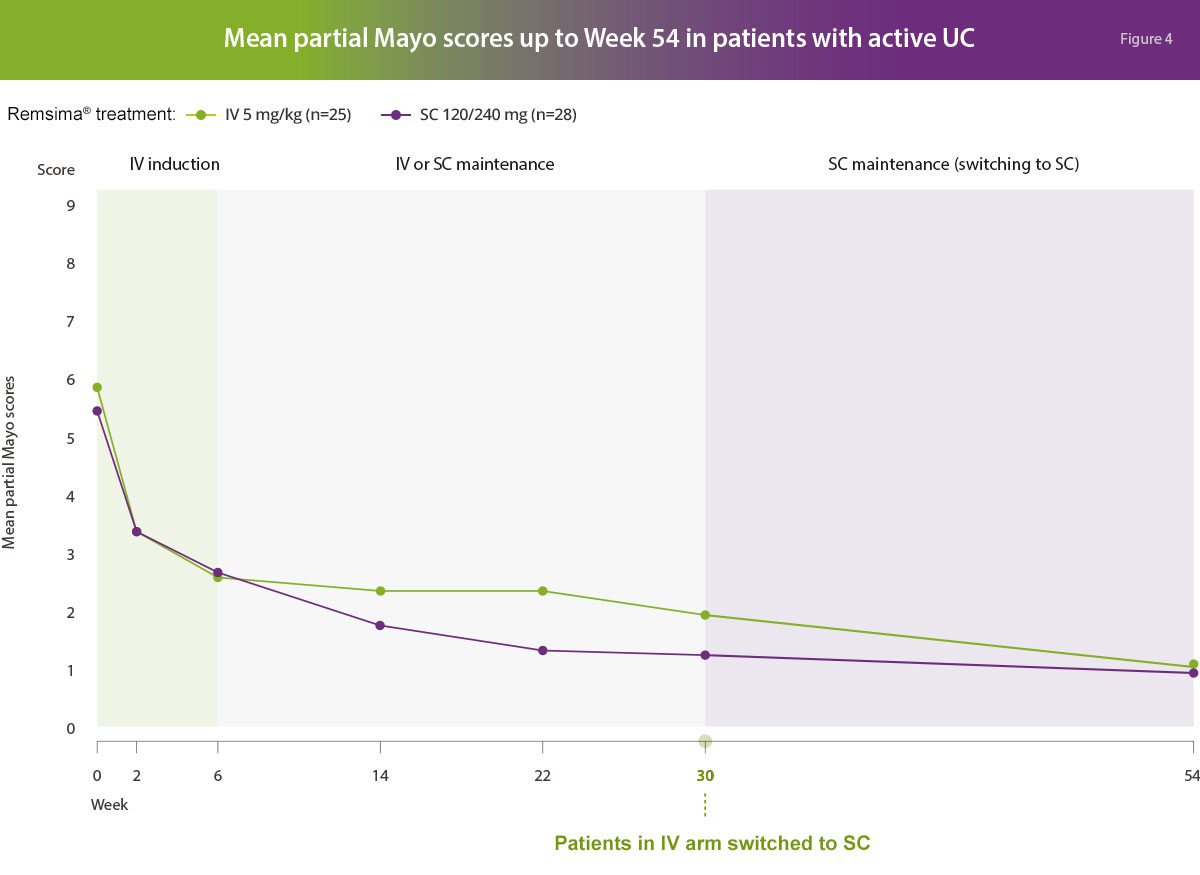
Adapted from Schrieber S, et al. Gastroenterology; accepted 2021.1
References
1. Schreiber S, Ben-Horin S, Leszczyszyn J, Dudkowiak R, Lahat A, Gawdis-Wojnarska B, Pukitis A, Horynski M, Farkas K, Kierkus J, Kowalski M, Lee SJ, Kim SH, Suh JH, Kim MR, Lee SG, Ye BD, Reinisch W. Randomized Controlled Trial: Subcutaneous vs Intravenous Infliximab CT-P13 Maintenance in Inflammatory Bowel Disease. Gastroenterology. 2021 Jun;160(7):2340-2353. doi: 10.1053/j.gastro.2021.02.068. Epub 2021 Mar 5. PMID: 33676969.
2. Remsima® Australian Product Information. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2020-PI-02540-1&d=202103141016933&d=20221121172310101. Last accessed: Nov 2022
Abbreviations
CD, Crohn’s disease; CDAI, Crohn's Disease Activity Index; IBD, inflammatory bowel disease; IV, intravenous; PK, pharmacokinetics; SC, subcutaneous; SD, standard deviation; SES-CD, Simplified Endoscopic Activity Score for Crohn’s Disease; UC, ulcerative colitis
Safety
The safety profile of Remsima® subcutaneous formulation in patients with active Crohn’s disease and active ulcerative colitis patients was overall similar to the safety profile of the intravenous formulation.1,2
There was no incidence of systemic injection reactions reported during this study. The incidence of localised injection site reactions (e.g. injection site erythema, pain, pruritus, bruising) was 28.1 per 100 patient-years in the Remsima® subcutaneous group (from Week 6). All of these reactions were mild to moderate and mostly resolved spontaneously without any treatment within a few days.1
References
1. Schreiber S, Ben-Horin S, Leszczyszyn J, Dudkowiak R, Lahat A, Gawdis-Wojnarska B, Pukitis A, Horynski M, Farkas K, Kierkus J, Kowalski M, Lee SJ, Kim SH, Suh JH, Kim MR, Lee SG, Ye BD, Reinisch W. Randomized Controlled Trial: Subcutaneous vs Intravenous Infliximab CT-P13 Maintenance in Inflammatory Bowel Disease. Gastroenterology. 2021 Jun;160(7):2340-2353. doi: 10.1053/j.gastro.2021.02.068. Epub 2021 Mar 5. PMID: 33676969.
2. Remsima® Australian Product Information. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2020-PI-02540-1&d=202103141016933&d=20221121172310101. Last accessed: Nov 2022.
Abbreviations
CD, Crohn’s disease; CDAI, Crohn's Disease Activity Index; IBD, inflammatory bowel disease; IV, intravenous; PK, pharmacokinetics; SC, subcutaneous; SD, standard deviation; SES-CD, Simplified Endoscopic Activity Score for Crohn’s Disease; UC, ulcerative colitis
Immunogenicity
In Crohn's disease and ulcerative colitis patients who received maintenance treatment of subcutaneous infliximab, the antibody incidence was not higher than in patients who received intravenous infliximab.1
References
1. Schreiber S, Ben-Horin S, Leszczyszyn J, Dudkowiak R, Lahat A, Gawdis-Wojnarska B, Pukitis A, Horynski M, Farkas K, Kierkus J, Kowalski M, Lee SJ, Kim SH, Suh JH, Kim MR, Lee SG, Ye BD, Reinisch W. Randomized Controlled Trial: Subcutaneous vs Intravenous Infliximab CT-P13 Maintenance in Inflammatory Bowel Disease. Gastroenterology. 2021 Jun;160(7):2340-2353. doi: 10.1053/j.gastro.2021.02.068. Epub 2021 Mar 5. PMID: 33676969.
2. Remsima® Australian Product Information. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2020-PI-02540-1&d=202103141016933&d=20221121172310101. Last accessed: Nov 2022.
Abbreviations
CD, Crohn’s disease; CDAI, Crohn's Disease Activity Index; IBD, inflammatory bowel disease; IV, intravenous; PK, pharmacokinetics; SC, subcutaneous; SD, standard deviation; SES-CD, Simplified Endoscopic Activity Score for Crohn’s Disease; UC, ulcerative colitis


