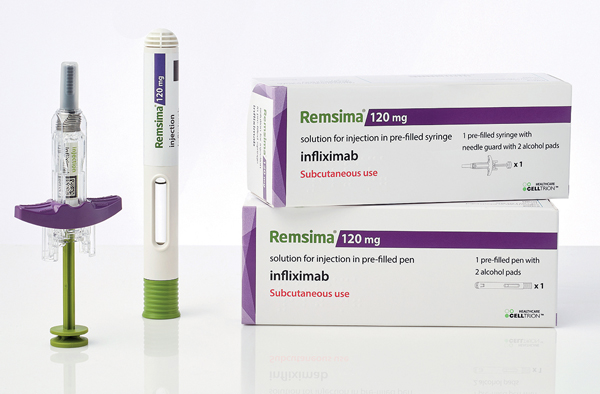
Presentations of Remsima® subcutaneous formulation.
Device Informantion
| Device perspective | Product perspective | |
|
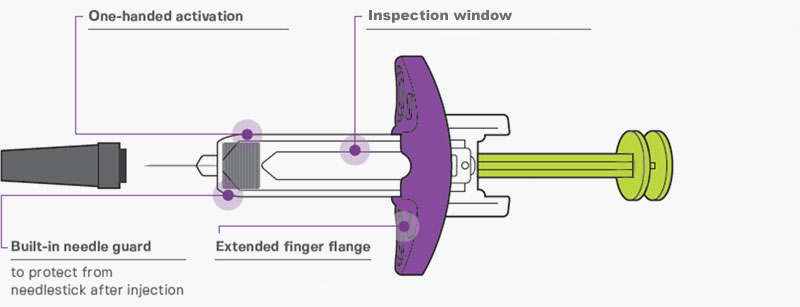
2-step operation pen Large inspection window / green plunger rod to show injection status Extended finger flange / ergonomic plunger head Built-in needle guard / automatic needle cover |
Stable up to 28 days outside the fridge for temperatures below 25oC1 |
|
Latex free1 |
||
|
29-gauge /5-bevel needle shown to reduce pain compared with 27-gauge needle2,3 |
Citrate free1, to reduce injection pain.4 The use of citrate as a buffer has been shown to cause more immediate pain after SC injection than a solution with histidine.4 |
Characteristics
2-STEP PROCESS
LATEX FREE1
29-GAUGE/5-BEVEL
CITRATE FREE1
STABLE UP TO 28 DAYS
outside the fridge1

Characteristics
PASSIVE ACTIVATION for patients who prefer manual injection control1
29-GAUGE/5-BEVEL
BUILT-IN NEEDLE GUARD to protect from needlestick after injection1
LATEX FREE1
CITRATE FREE1
STABLE UP TO 28 DAYS
outside the fridge 1
Authority required. Refer to PBS Schedule for full authority information.
References
1. Remsima® Australian Product Information. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2020-PI-02540-1&d=202103141016933&d=20221121172310101. Last accessed: Nov 2022.
2. Glenski, Stephen, and Jill Conner. “29 gauge needles improve patient satisfaction over 27 gauge needles for daily glatiramer acetate injections.” Drug, healthcare and patient safety vol. 1 (2009): 81-6. doi:10.2147/dhps.s8495
3. Jaber, Amer et al. “A novel needle for subcutaneous injection of interferon beta-1a: effect on pain in volunteers and satisfaction in patients with multiple sclerosis.” BMC neurology vol. 8 38. 10 Oct. 2008, doi:10.1186/1471-2377-8-38
4. Laursen T, et al. “Pain perception after subcutaneous injections of media containing different buffers.” Basic Clin Pharmacol Toxicol 2006;98(2):218-21, doi: 10.1111/j.1742-7843.2006.pto_271.x.
Abbreviation
SC, subcutaneous



 Click
Click Click
Click